Achieve Wellness With PEMF Therapy
PEMF therapy treats inflamed and painful areas safely and with no known undesirable side effects or down time. The magnetic pulses penetrate the tissue layers and induce muscle relaxation, lower nerve irritability as well as stimulate blood and lymph circulation. In doing so, PEMF therapy reduces pain, swelling and inflammation, promotes cellular and nerve regeneration, strengthens bones, and accelerates fracture repair. PEMF therapy improves cellular membrane permeability as the pulses mechanically contract the cell walls and activate the ion (Sodium / Potassium) pump by opening and closing the ion channels through the membrane. More oxygen and nutrients enter the cells, and more carbon dioxide and waste products are carried out of the cells. PEMF therapy stimulates overall tissue healing and immune system response.
Primary Benefits of PEMF Therapy
Clinical evidence demonstrates that PEMF therapy reduces pain associated with wounds or trauma from accidents, sports injuries, surgeries and burns. PEMF therapy may improve disease and degeneration response.
In 1995, Siskin and Walker provided a summary of clinical results on soft tissue damage. They observed no adverse effects and reported the following positive effects:
• Reduced pain
• Reduced inflammation
• Increased range of motion
• Faster functional recovery
• Reduced muscle loss after surgery
• Increased tensile strength in ligaments
• Faster healing of skin wounds
• Enhanced capillary formation
• Accelerated nerve regeneration
• Reduced tissue necrosis.
In the “Beneficial effects of electromagnetic fields”, Bassett C. (Bioelectric Research Center, Columbia University, NY, 1993) applied time-varying pulsed magnetic fields to induce voltages similar to those normally produced during the dynamic mechanical deformation of connective tissues to control cellular function and understand the mechanisms by which PEMF treatment operates. He concludes: “As a result, a wide variety of challenging musculoskeletal disorders has been treated successfully over the past two decades... Many of the athermal bio responses, at the cellular and subcellular levels, have been identified and found appropriate to correct or modify the pathologic processes for which PEMFs have been used… As understanding of mechanisms expands, specific requirements for field energetics are being defined and the range of treatable ills broadened. These include nerve regeneration, wound healing, graft behavior, diabetes, and myocardial and cerebral ischemia (heart attack and stroke), among other conditions. Preliminary data even suggests possible benefits in controlling malignancy”.
PEMF Therapy and Nitric Oxide Production
Many cells in the body produce nitric oxide, a radical gas that is a key vertebrate biological messenger playing a role in many biological processes. The vascular endothelium production of nitric oxide is particularly important in the regulation of blood flow. Abnormal production of nitric oxide, as occurs in different disease states, can adversely affect blood flow and other vascular functions.
The March/April 2009 Aesthetic Surgery Journal published a study: “Evidence-Based Use of Pulsed Electromagnetic Field Therapy in Clinical Plastic Surgery” that summarizes the effects of PEMF therapy on cells and tissues. Studies suggesting that PEMF could modulate the production of growth factors emerged and began to focus on enzyme systems with well-characterized calcium (Ca2+) dependence. By the mid-1990s, researchers were investigating the effects of electrical and PEMF signaling on intracellular Ca2+, specifically the binding of Ca2+ to Calmodulin (CaM) with the knowledge that CaM dependent cascades were involved in tissue repair. The most recent studies on the PEMF transduction pathways concentrate on the Ca/CaM-dependent nitric oxide cascades, the growth factor cascades involved in tissue healing. It is within this system that the effectiveness of PEMF is now understood to function. PEMFs modulate the calcium-binding kinetics to Calmodulin. Calcium/Calmodulin (Ca/CaM) then activates nitric oxide synthase (NOS) in several different isoforms.
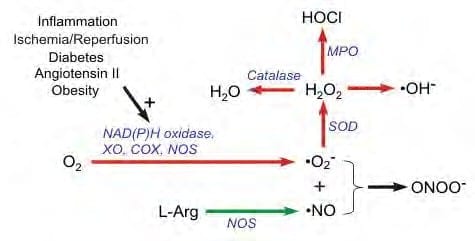
When injury occurs, large amounts of nitric oxide are produced by long-lived inducible nitric oxide synthase (iNOS). In this cascade, tissue levels of nitric oxide persist and the prolonged presence of this free radical is pro-inflammatory. This accounts for the leaky blood vessels syndrome associated with pain and swelling. In contrast, the endothelial and neuronal nitric oxide synthase isoforms (respectively eNOS and nNOS) produce nitric oxide in short bursts that can immediately relax blood and lymph vessels. These short bursts of nitric oxide also lead to the production of cyclic Guanosine Monophosphate (cGMP), which in turn drives growth factor production. Interestingly, iNOS is not dependent on CaM, while the constitutive nitric oxide synthase or cNOS (eNOS or nNOS) cascade is dependent on the binding of Ca/CaM. Therapies, such as PEMF therapy that can accelerate Ca/CaM binding may therefore impact all phases of tissue repair, from initial pain and swelling to blood vessel growth, tissue regeneration and remodeling as shown in the following diagram, this mechanism has been proposed as a working model for PEMF therapeutics. NOS, also known as the endothelium-derived relaxing factor, or 'EDRF', is bio-synthesized endogenously.
In addition, the endothelial neural NOS (nNOS, Type I) serves as a transmitter in the brain and in different nerves of the peripheral nervous system to stimulate vasodilatation. The endothelium (inner lining) of blood vessels uses NO to signal the surrounding smooth muscles to relax, thus increasing vasodilatation and blood flow.

Under normal conditions, NO is continually being produced by cNOS in the blood vessels. The Ca/CaM-dependent activity of cNOS produces vascular relaxation only when the endothelium is intact.
Under normal conditions, the Ca independent activation of iNOS is very low. Bacterial endotoxins or cytokines, such as tumor necrosis factor (TNF) and interleukins stimulate the activity of iNOS and thus cause inflammation.
During inflammation, the amount of NO produced by iNOS may be a 1,000-fold greater than that produced by cNOS. When NO is formed, it is highly reactive (having a lifetime of a few seconds), yet diffuses freely across membranes, primarily because superoxide anion has a high affinity for NO. Superoxide anion and its products have tissue damaging effects and can have vasoactive properties. Superoxide anion is thus very important in cardiovascular pathology and pathophysiology. Its unpaired electron binds very rapidly to NO, which also has an unpaired electron. The reaction between NO and superoxide anion effectively scavenges NO and reduces its bioavailability leading to vasoconstriction, increased platelet-endothelial cell adhesion, platelet aggregation and thrombus formation, increased leukocyte - endothelial cell adhesion, and morphologic changes in blood vessels, including cell proliferation.
NO also avidly binds to the hemoglobin (Hgb) in red blood cells and the enzyme guanylyl cyclase, which is found in vascular smooth muscle cells and most other cells of the body. When NO is formed by the vascular endothelium, it rapidly diffuses into the blood where it binds to Hgb breaking it down.
It also diffuses into the smooth vascular muscle cells adjacent to the vascular endothelium where it binds to and activates guanylyl cyclase.
This enzyme catalyzes the dephosphorylation of Guanosine Triphosphate (GTP) to Cyclic Guanosine Monophosphate (cGMP), which serves as a second messenger for many important cellular functions and, in particular, for signaling smooth muscle relaxation.
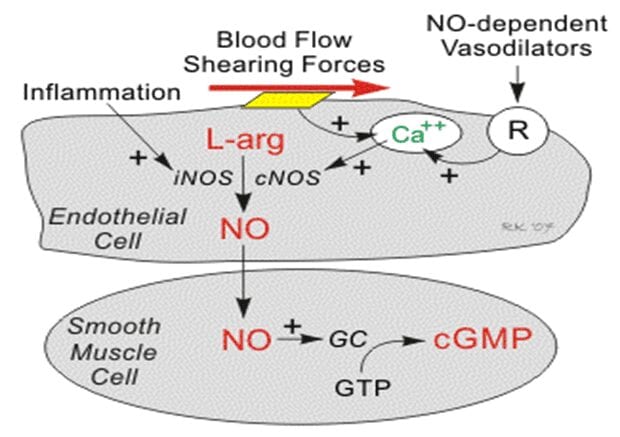
Drugs that are GMP-dependent phosphodiesterase inhibitors (e.g., Viagra®) inhibit the breakdown of cGMP and are used to enhance NO mediated vasodilation, particularly in penile tissue to treat erectile dysfunction. Increased cGMP has also an important anti-platelet, anti-aggregatory effect. (Cardiovascular Physiology
Concepts by Richard E. Klabunde, PhD, published in 2005, www.cvphysiology.com, 2008). In a study entitled “Pulsed Electro-Magnetic Fields Affect Local Factor Production and Connexin 43 Protein Expression in MLO-Y4 Osteocyte-like cells and ROS17/2.8 Osteoblasts like Cells ”, Lohman C.H. et Al. state: “This study shows that PEMFs affect gap junction formation, local production NO, TGF-β1 and PGE2. Osteocytes can potentially regulate bone remodeling through signaling molecules like NO and PGE2 but also through the local release of TGF-β1.”
The above studies demonstrate that PEMF therapy affects many transduction pathways and, in particular the Ca/CaM-dependent nitric oxide cascades involved in tissue repair. By modulating intracellular Ca /CaM, the eNOS and nNOS produce NO in short bursts that can immediately relax blood and lymph vessels. As a highly reactive gaseous molecule, NO is an ideal transient paracrine (between adjacent cells) and autocrine (within a single cell) signaling molecule that has direct and indirect vascular action, including the following:
- Direct vasodilatation (flow dependent and receptor mediated)
- Indirect vasodilatation by inhibiting vasoconstrictor influences
- Anti-thrombotic effect - inhibits platelet adhesion to the vascular endothelium
- Anti-inflammatory effect - inhibits leukocyte adhesion to vascular endothelium; scavenges superoxide anion
- Anti-proliferative effect - inhibits smooth muscle hyperplasia.
By increasing the production of NO when its production is impaired or its bioavailability is reduced, PEMF therapy can successfully help improve conditions and diseases associated with vasoconstriction (e.g., coronary vasospasm, elevated systemic vascular resistance, hypertension), thrombosis due to platelet aggregation and adhesion to vascular endothelium, inflammation by up regulating leukocyte and endothelial adhesion molecules, vascular hypertrophy , stenosis, hypertension, obesity, dyslipidemias (particularly hypercholesterolemia and hypertriglyceridemia), diabetes (both type I and II), atherosclerosis, heart failure, tissue repair and aging.
A recent study on postoperative recovery led to the conclusion that PEMF therapy significantly reduces postoperative pain and narcotic use in the immediate postoperative period by the PEMF effect on NO signaling, which could impact the speed and quality of wound repair (Rohde et Al. June 2009, Plastic & Reconstructive Surgery, Columbia, NY).
Other mechanisms by which NO has been demonstrated to affect the biology of living cells are numerous and include: oxidation of iron-containing proteins such as ribonucleotide reductase and aconitase; activation of the soluble guanylate cyclase, a transmembrane protein; ADP (adenosine di-phosphate) ribosylation of proteins, a process of protein modification involved in cell signaling; DNA repair; protein sulfhydryl group nitrosylation, another protein modification process; and iron regulatory factor activation.
With a lifetime of a few seconds, NO is highly reactive and diffuses freely across cell membranes. As PEMF therapy effectively stimulates NO production, it also improves paracrine and autocrine communication. NO is also generated by phagocytes (monocytes, macrophages, and neutrophils) and, as such, is part of the human immune response.
NO has been demonstrated to activate NF -κB in peripheral blood mononuclear cells, an important protein complex that controls the transcription of DNA and a transcription factor in iNOS gene expression in response to inflammation.
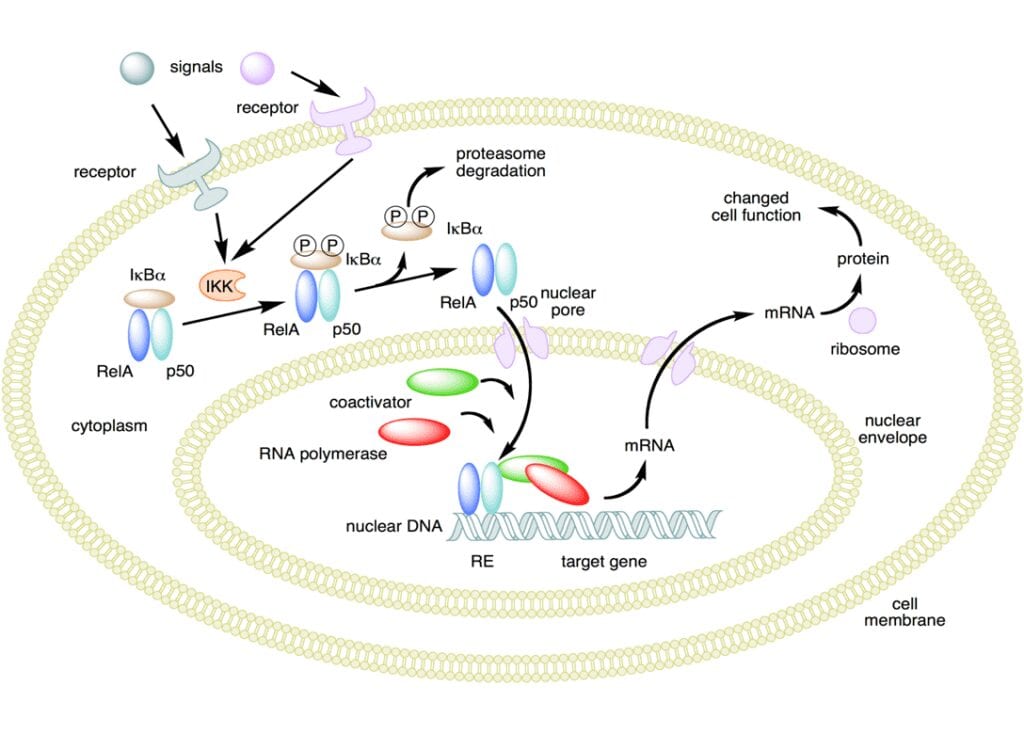
NO plays a key role in regulating the immune response to infection and is implicated in processes of synaptic plasticity and memory (see diagram above). The endothelium (inner lining) of blood vessels uses nitric oxide to signal the surrounding smooth muscle to relax, thus resulting in vasodilatation and increasing blood flow. As blood flow increases, so does the oxygen intake. PEMF therapy has proven to effectively increase blood flow and provide muscle relaxation with better oxygenation of the muscle tissue.
The Dynamics of Pain and PEMF Therapy
One of the most significant effects of PEMF therapy is the improvement of painful conditions regardless of their origin. Pain mechanisms are complex and have peripheral and central nervous system aspects.
During the last 100 years, theories of pain mechanism have evolved from specificity and summation models to the popular Gate Control Theory. The latter pain theory, proposed by Melzack/Wall/Casey (Wall and Melzack, 1989) has become the most important development in the field of pain management.
In biology, signal transduction is a mechanism that converts a mechanical or chemical stimulus to a cell into a specific cellular response. Signal transduction starts with a signal to a receptor, and ends with a change in cell behavior. Transmembrane receptors move across the cell membrane, with half of the receptor outside and the other half inside the cell. The signal, such as a chemical signal, binds to the outer half of the receptor which then changes its shape thus conveying another signal inside the cell. There may be a long cascade of signals, one after the other. The signal creates a change in the cell, either in the DNA of the nucleus or the cytoplasm outside the nucleus. In a chronic pain state, the pain signal generated can actually occur in the central nervous system without any peripheral noxious stimulation.
PEMF modulates the pain signal transmission. Scientific evidence shows that acute persistent pain eventually sensitizes wide dynamic neurons in the dorsal horn of the spinal cord, the wind-up phenomenon, constituting the basis of developing chronic pain syndromes (Kristensen, 1992). Persistent and excessive pain has no biological good or necessary function. It is actually harmful to our well-being. Therefore, pain needs to be treated as early and as completely as possible and not to be left alone (Adams et Al. 1997).
The primary symptom in most patients with disorders affecting the soft tissue is pain from local inflammation. Causes of soft tissue pain can be depicted as musculo - skeletal, neurologic, vascular, and referred visceral - somatic or articular (Cailliet, 1991). In many patients, inflammation causes pain that limit range of movements and daily activities. Early reports of applying electrical current to treat pain date back to before 1800 (Ersek, 1981).
PEMF therapy has successfully been used for t he control of pain associated with rotator cuff tendinitis, multiple sclerosis, carpal tunnel syndrome, and peri -arthritis (Battisti et Al. 1998; Lecaire et Al. 1991). PEMF therapy has also successfully been used for treatment of migraine, chronic pelvic pain, neck pain, and whiplash injuries (Rosch et Al. 2004).
In a March 2003 publication on Pain Management with PEMF Treatment, Dr. William Pawluk explains:”Magnetic fields affect pain perception in many different ways. These actions are both direct and indirect. Direct effects are: neuron firing, calcium ion movement, membrane potentials, endorphin levels, nitric oxide, dopamine levels, acupuncture actions and nerve regeneration. Indirect benefits are on: circulation, muscle, edema, tissue oxygen, inflammation, healing, prostaglandins, cellular metabolism and cell energy levels… Short -term effects are thought due to a decrease in cortisol and noradrenaline, and an increase in serotonin, endorphins and enkephalins. Longer term effects may be due to CNS and/or peripheral nervous system biochemical and neuronal effects in which pain messages are altered; and the pain is not just masked as in the case of medication”.
- PEMF Therapy Reduces Pain
Many studies have demonstrated the positive effects of PEMF therapy on patients with pain from a wide variety of conditions. Some studies focused on the rapid short- term relief while others demonstrate the long-term benefits.
In “Double-blind, placebo-controlled study on the treatment of migraine with PEMF”, Sherman et Al. (Orthopedic Surgery Service, Madigan Army Medical Center, Tacoma, WA, USA) treated 42 subjects who met the International Headache Society's criteria. After the first month of PEMF, 73% of the active group reported decreased headaches of which 45% a substantial decrease and 14% an excellent decrease. Half the active group received two additional weeks of treatment. All showed decreased headache activity with 50% a substantial decrease and 38% an excellent decrease. Sherman et Al. concluded that PEMF treatment for at least 3 weeks is an effective, short -term intervention for migraine.
Jorgensen et Al. (1994 International Pain Research Institute, Los Angeles, CA, USA) studied the effects of PEMF on tissue trauma in patients with cases such as ruptured ovarian cyst, endometriosis, dyspareunia or post-operative hematoma who had not received analgesic medication. Ninety percent experienced marked, even dramatic relief, while 10% reported less than complete pain. “Unusually effective and long - lasting relief of pelvic pain of gynecological origin has been obtained consistently by short exposures of affected areas to the application of a magnetic induction device. Treatments are short, fa sting-acting, economical, and in many instances have obviated surgery”.
Hedén P., Pilla AA. (2008 Department of Plastic Surgery, Stockholm, Sweden) studied the effects of PEMF on postoperative pain in breast augmentation patients and notes: “Postoperative pain may be experienced after breast augmentation surgery despite advances in surgical techniques that minimize trauma. The use of pharmacological analgesics and narcotics may have undesirable side effects that can add to patient morbidity”. Postoperative pain data showed pain had decreased in the treated group by nearly a factor of three times that of the control group. Patient use of postoperative pain medication also decreased nearly three times faster in the active versus the control group. Hedén P Pilla AA. concluded: “Pulsed electro-magnetic field therapy, adjunctive to standard of care, can provide pain control with a noninvasive modality and reduce morbidity due to pain medication after breast augmentation surgery”.
The Clinical Rheumatology Journal, volume 26 -1, January 2007 (Springer London) published a study by Kaan Uzunca, Murat Birtane and Nurettin Taştekin (Trakya University Medical Faculty Physical Medicine and Rehabilitation Department, Edirne, Turkey ): “We aimed to investigate the efficacy of PEMF in lateral epicondylitis comparing the modality with sham PEMF and local steroid injection”. One of three groups received PEMF, another sham PEMF and the third a corticosteroid + anesthetic agent injection. Pain levels during nighttime, rest, activity, resistance in wrist dorsiflexion and forearm supination were investigated with a visual analog scale. Pain threshold on elbow was determined with an Algometer. All patients were evaluated before treatment, at the third week and the third month. Pain levels were significantly lower in the steroid group at the third week but , at the third month, the PEMF group had lower pain during nighttime, rest and activity than the group receiving local steroids.
Lau (School of Medicine, Loma University, USA) reported on PEMF therapy and diabetic retinopathy. Patients were treated for 6 weeks, 76% showed a reduction in numbness and tingling. All patients experienced pain reduction and 66% reported being totally pain-free.
Sanseverino et Al. (Universita di Bologna, Italy 1999) studied the therapeutic effects of PEMF on joint diseases on chronic and acute conditions in 3,000 patients followed over 11 years. Pain control, recovery of joint mobility and maintenance of the improved conditions were evaluated as good or poor. The chi- square test was applied to eliminate casual results. An average value of 78.8% with good results and 21.2% with poor results was obtained. Sanseverino et Al. concluded that PEMF treatment is an excellent physical therapy for joint diseases with no undesired side -effects and hypothesized that external magnetic field may influence trans-membrane ionic activity.
In 2008, a randomized clinical trial on patients with carpal tunnel syndrome using a combination of static and time-varying dynamic magnetic field stimulation on the wrist. Weintraub et Al. report: “PEMF exposure in refractory carpal tunnel syndrome provides statistic ally significant short- and long-term pain reduction and mild improvement in objective neuronal functions”.
In a 2009 evidence-based analysis on the use of PEMF therapy in clinical plastic surgery, Strauch et Al. (Einstein College of Medicine, Bronx, NY, U SA) explain:” Our objective was to review the major scientific breakthroughs and current understanding of the mechanism of action of PEMF therapy… The results show that PEMF therapy has been used successfully in the management of postsurgical pain and edema, the treatment of chronic wounds, and in facilitating vasodilatation and angiogenesis… non-invasive with no pharmacologic management, no known side effects … what has been of most significance to the plastic surgeon is the laboratory and clinical confirmation of decreased pain and swelling following injury or surgery”.
PEMFs can affect pain perception in many different ways.
- PEMF Therapy Blocks Pain
PEMF therapy has shown to be effective at reducing pain both in the short-term and in the long-term. The ways by which PEMF therapy relieves pain include pain blocking, decreased inflammation, increased blood and fluids circulation and increased tissue oxygenation.
The trans-membrane potential (“TMP”) is the voltage difference between the interior and exterior of a cell. It is an electrochemical gradient that results from a spatial variation of both an electrical potential and a chemical concentration across a membrane. These are often due to ion gradients, particularly proton gradients, and the result is a type of potential energy available for cellular metabolism as evaluated by thermodynamic measure.
Differences in concentration of ions on opposite sides of a cell membrane produce the TMP.
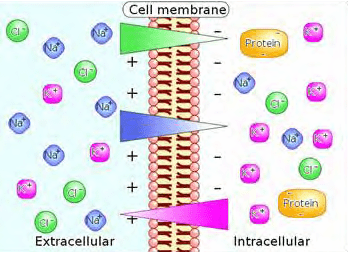
Sodium (Na+) and chloride (Cl–) ions are in high concentrations in the extracellular region while potassium (K+) ions and large protein anions are in high concentrations in the intracellular region. The opening and closing of ion channels to transport ions (Na+, Ca2+, K+, Cl-) in and out of cells through the membrane produce a local change in the transmembrane potential (“TMP”) which causes a rapid flow of electrons to other areas of the membrane, thus generating an electric current. In electrically excitable cells such as neurons, the TMP is used to transmit signals from one part of another.
In their baseline states, both non-excitable and excitable cells have a TMP at a relatively stable value called the resting potential. The interior of a cell has a negative baseline voltage relative to the outside. Neurons have typical resting potential values ranging from -70 to -80 mV (mill Volts) and each axon has its characteristic resting potential voltage. The opening and closing of the ion channels induce a change in the resting potential, called a depolarization if the interior voltage rises (say from -70 mV to -65 mV), or a hyper polarization if the interior voltage decreases (say from -70 mV to -80 mV).
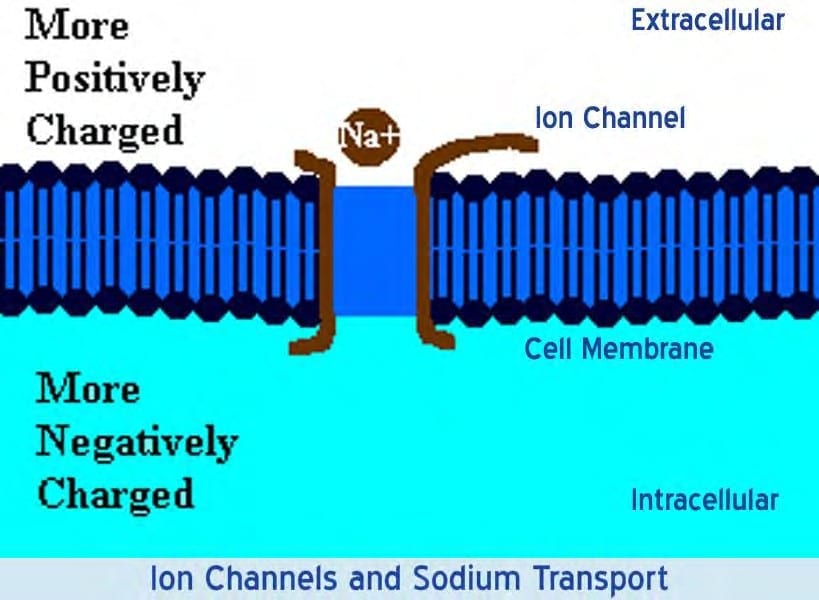
In excitable cells, a sufficiently large depolarization evokes a short-lasting, all-or-nothing event called an action potential where the TMP undergoes a sudden, substantial but extremely brief change, a spike often reversing its sign. Special types of voltage -dependent ion channels that remain closed at the resting TMP can be induced to open by a small depolarization.
Dr. D. Laycock, Ph.D. Med. Eng. MBES, MIPEM, B.E d., inspired by the works of Adams et Al. (1997) lectured on PEMF therapy and Pain Reduction: “It is necessary to understand the mechanism of pain transmission to understand how pain blocking can take place with PEMF therapy...” Pain is transmitted first by an electric signal along the nerve cells to pre -synaptic terminals, then by a chemical signal across synaptic gaps between neurons…
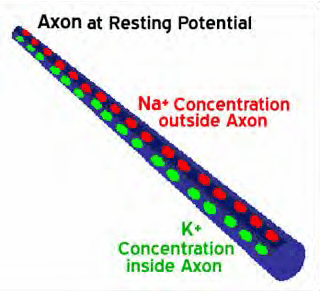
During quiescent times, cells possess a TMP of –70mV between the inner and outer membrane. The Na+/K+-ATPase helps maintain resting potential. When a pain signal arrives, it temporarily depolarizes the nociceptive cell and raises the TMP to +30mV. This rise opens channels in the membrane allowing the exchange of the sodium (Na+) and potassium (K+) ion s. In the depolarization phase, the voltage inside the axon is positive relative to the outside and the concentration of Na+ ions inside the axon becomes greater.
The Na+ channels close when the inside of the axon becomes sufficiently positive, about +30 mV. This closing of the Na+ channels limits the ability of Na+ ions to enter the axon. Now K+ ions are free to cross the channels and leave the axon because of the greater concentration of K+ on the inside and the reversed voltage levels. The action potential is therefore not the movement of voltage or ions but the flow of these ion channels opening and closing moving down the axon. This exchange of Na+ and K+ triggers exocytosis of neurotransmitters via synaptic vesicles contained within the membrane. The neurotransmitters diffuse into the synaptic gap and chemically transfer the pain signal across the synaptic gap to chemical receptors on the post-synaptic nerve cell. The cell then returns to its previous level of –70mV.
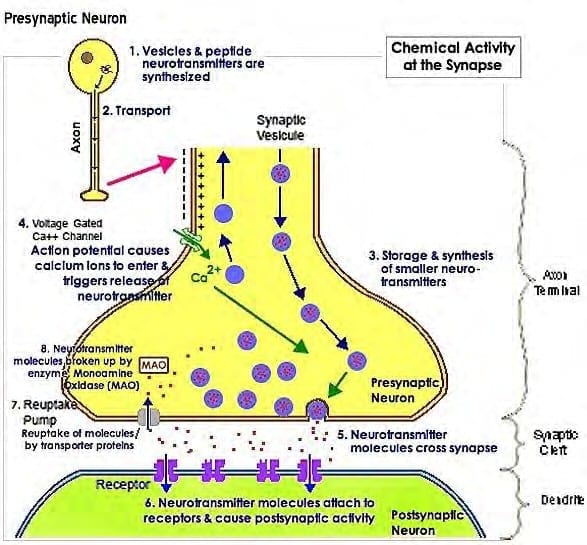
“This all happens in about 1/2000th of a second, as the synaptic gap is only 20 to 50 nm (nanometers) wide...”
The mechanical movement of opening and closing the ion channels explains why, unlike the normal flow of electrons in an electrical field which travel at the speed of light, the action potential is transferred slowly.
Warnke et Al. (1997) suggest that the PEMF effect is to lower the TMP to a hyperpolarized level of –90mV thus preventing pain transmission: “When a pain signal is received, the TMP has to be raised in order to f ire an action potential via neurotransmitters but it only raises the cell TMP to an approximate +10mV. This potential is well below the threshold of +30mV necessary to release the relevant neurotransmitters into the synaptic cleft and the pain signal is effectively blocked”.
In the same way, PEMF therapy effectively increases the TMP of damaged cells thus allowing them to heal, improve their metabolism and recover their functions. The Encyclopedia of Nursing and Allied Health define the use of “Electrotherapy” for pain relief as effective to manage both acute and chronic pain. In the “Gate Model” of pain suggests that intense stimulation of proprioception fibers can block the slower - moving pain signals.
PEMF Therapy Reduces Inflammation
Several factors contribute to inflammation including injury, tissue damage, surgery or simply poor localized circulation. The formation of edema with swelling, bruising and discoloration of soft tissue is an inflammatory response. Inflammation causes pain.
Tissue cells are inherently like tiny electrically charged batteries. When a cell is traumatized, the cell’s electrical charge is diminished; this causes normal cell functions and operations to shut down. Cells that are scarred or fibrotic with adhesions have a TMP charge of about -15 mV, degenerative or immune -compromised cells about -30 mV. With raised resting TMP, the cells release chemical signals that cause inflammation, swelling, bruising and, subsequently, pain. Communication pathways necessary for healing to begin are interrupted.
Numerous clinical studies have demonstrated that PEMF therapy reduces inflammation. PEMF therapy provides a mild electromagnetic current that effectively recharge cells and stop the release of inflammatory free-radicals (iNOS). With reduced inflammatory fluids released, blood flow increases as does oxygen intake, helping cells heal faster with less swelling, bruising and pain.
Jasti et Al. (2001) studied the effect of wound healing electromagnetic fields on inflammatory cytokine gene expression in rats and state: “Inflammation is characterized by massive infiltration of T lymphocytes, neutrophils and macrophages into the damaged tissue. These inflammatory cells produce a variety of cytokines, which are the cellular regulators of inflammation”.
Ganesan et Al. (Department of Biotechnology, Chennai, India, 2009) studied Low Frequency PEMF as a viable alternative therapy for arthritis: “PEMF for arthritis cure has conclusively shown that PEMF not only alleviates the pain in the arthritis condition but it also affords chondroprotection, exerts anti -inflammatory action and helps in bone remodeling, and this could be developed as a viable alternative for arthritis therapy”.
Damaged cells have a reduced electrical charge and are energy deficient. They have low oxygen and high sodium levels causing a faltering electrochemical gradient. PEMF therapy induces a mild electrical current into damaged cells that slows or stops the release of pain and of inflammatory signaling molecules, increases blood flow, and stimulates normal cell function. The cell starts pumping Na+ out and K+ in restoring the electrochemical gradient; oxygen starts flowing back in, swelling resorbs and pain decreases. The most effective PEMF restore the cells TMP to its optimal healthy -70 mV.
M. Fni. G. Giavaresi, A. Carpi, A. Nicolini, S. Setti, R. Giardino (Experimental Surgery Department, Research Institute Codivilla-Putti-Rizzoli, Orthopedic Institute, Bologna, Italy, Department of Reproduction and Aging, University of Pisa, Italy, Department of Internal Medicine, University of Pisa, Italy, Igea SRL, Carpi, Modena, Italy) report in the Elsevier Journal of Biomedicine & Pharmacotherapy 2005 publication on the Effects of pulsed electromagnetic fields on articular hyaline cartilage: “Newer concepts on osteoarthritis (OA) pathogenesis are related to the role of inflammation that is now well accepted as a feature in OA. Synovitis is common in advanced age involving infiltration of activated B cells and T lymphocytes. The expression of pro-inflammatory cytokines and chemokines is observed in the joints of OA patients and animals.... A therapy combining an anabolic effect on chondrocytes, a catabolic cytokine blockage, a stimulatory effect on anabolic cytokine production and one that is able to counteract the inflammatory process would be extremely useful for OA treatment. In vitro studies showed that chondrocyte proliferation and matrix synthesis are significantly enhanced by PEMF stimulation... The importance of physical properties of the fields used (intensity, frequency, impulse amplitude, etc.) and the exposure time, the availability of growth factors, environmental constrictions and the maintenance of the native–cell matrix interactions seem to be fundamental in the PEMF-induced stimulation…
With inflammation, IL-1a is present in high amounts in OA cartilage and is considered to be one of the main catabolic factors involved in the cartilage matrix degradation associated with OA … PEMFs in vitro were able to counterbalance efficiently the cartilage degradation induced by the catabolic cytokine” .
The studies above demonstrate how PEMF therapy is effective and reduces inflammation. The mechanisms by which PEMF therapy operates to reduce inflammation include complex mechanical, chemical and electromagnetic processes.
PEMF Therapy Increases Blood and Lymphatic Circulation
PEMF therapy mechanically stimulates blood vessels and blood flow. The blood vessels pump blood and oxygen into the cells. PEMF therapy also mechanically stimulates the lymphatic vessels and waste products are hauled away from the cells more efficiently. PEMF therapy supports immune health by mechanically stimulating lymphatic drainage and blood flow.
In June 2004, The Faseb Journal states: “PEMF therapy has been shown to be clinically beneficial in repairing bones and other tissues, but the mechanism in action is unclear”. A study done at the New York University Medical Center (Institute of Reconstructive Plastic Surgery, NY, NY, USA) demonstrates: “Electro-magnetic fields increases angiogenesis, the growth of new blood vessels, in vitro and in vivo, through the endothelial release of FGF-2, fibroblast growth factor-2. The delivery of PEMF therapy in low doses identical to that currently in clinical use significantly increased endothelial cell proliferation and tubulization, which are both important processes for vessel formation.
The ability of PEMF to increase cell proliferation was unique to endothelial cells, releasing a protein in a paracrine fashion (or signaling to adjacent cells and other types of cells) to induce changes in neighboring cells and tissues. Since direct stimulation did not produce significant changes in osteoblast proliferation, the ability of PEMF therapy to enhance the healing of complicated fractures is likely the result of increased vascularity rather than of a direct effect on osteogenesis as previously believed. The coordinated release of FGF - 2 suggests that PEMF therapy may facilitate healing by augmenting the interaction between osteogenesis and blood vessel growth.
In a study, “Impulse magnetic-field therapy for erectile dysfunction: a double-blind, placebo-controlled study”, Pelka et Al. (Universitat der Bundeswehr Munchen, Munich, Germany) assessed the efficacy of three weeks of PEMF therapy for erectile dysfunction. They stated: “By increasing microcirculation, impulse magnetic -field therapy leads to improvements in macro -circulation. At study end, all efficacy endpoints were significantly improved with 80% reporting increases in intensity and duration of erection, frequency of genital warmth, and general well-being in the active-treatment group. In contrast, only 30% of the placebo group noted some improvement in their sexual activity; 70% had no change. No side effects were reported”.
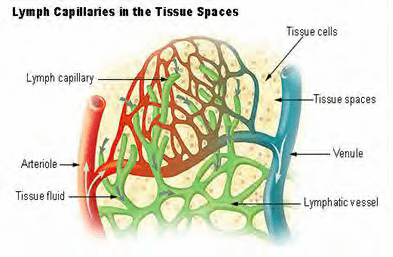
Many studies show PEMF therapy increases the flow of ions and nutrients into the cells and stimulates blood and interstitial fluid circulation.
With increased lymphatic drainage and blood flow, cells receive more oxygen and nutrients, an d eliminate toxins faster. Cells are then able to function better and tissues repair themselves more efficiently. Vital organs such as the liver, kidneys and colon are able to rid themselves of impurities thus detoxifying the body and allowing better organ functionality.
PEMF Therapy Increases Cell Membrane Permeability
As early as 1940, it was suggested that magnetic fields affect the TMP and the flow of ions in and out of the cells, and therefore cell membrane permeability. It has since been established that magnetic fields do influence ATP (Adenosine Tri-phosphate) production; increase the supply of oxygen and nutrients via the vascular and lymphatic systems; improve the removal of waste via the lymphatic system; and help re-balance the distribution of ions across the cell membrane. Healthy cells in tissue have a voltage difference between the inner and outer membrane referred to as the membrane resting potential that ranges from -70 to -80 mV. This causes a steady flow of ions through its volt age- dependent ion channels. The membrane potential of a damaged cell is raised and an increased sodium inflow occurs. As a result, interstitial fluid is attracted to the inner cellular space. PEMF accelerates the re- establishment of normal potentials increasing the rate of healing and reducing swelling (Sanseverino, 1999).
In biology, depolarization is a change in a cell's TMP, making it more positive or less negative, for example rising to -50mV from a resting potential of -70mV. In neurons and some other cells, a large enough depolarization may result in an action potential. Hyper polarization is the opposite of depolarization and it inhibits the rise of an action potential. Depolarization is caused by influx of cations, e.g. Na+ through Na+ channels, or Ca2+ through Ca2+ channels. On the other hand, an efflux of K+ through K+ channels or of the anion Cl– through Cl– channels inhibits depolarization. As the magnetic field created fluctuates, it induces an electron flow or a current in one direction through the living tissue. As electrons always flow from a negative (cathode) to a positive (anode) potential, when the magnetic field vanishes, the direction of the electron flow is reversed. Induced polarized currents stimulate the exchange of ions across the cell membrane. The electro-magnetic field pulses temporarily hyperpolarize and depolarize the membrane thus increasing cellular oxygenation and nutrition. Sodium export stimulates several secondary active transporters.
Increased cell membrane permeability allows better oxygen and nutrient uptake by the cells and increased toxins release from the cells. Cells are therefore better equipped to repair themselves and regenerate thus improving all their cellular functions and genesis. With increased cell membrane permeability there is an increased absorption of the chemicals available in the blood and lymphatic fluids surrounding a tissue area treated. As demonstrated by many medical studies, this phenomenon can be quite significant in the treatment cancerous tumors and localized cancers.
Cancer and Cellular Proliferation
As with all forms of new therapy, the incidence of negative side effects is a major concern. Since PEMF therapy is known to stimulate cellular genes is, a major concern is that it might stimulate metastasis of cancerous cells and tumors in cancer patients.
In a study entitled: “Effect of Pulsing Electromagnetic Fields on Invasion Ability in Osteosarcoma Cells.” in the 1999 Journal of the Japanese Bio -Electrical Research Society, Kobayashi Kenji et Al. (Nagoya University, Japan) declare: “We have been trying to apply pulsing electromagnetic fields (PEMF) to chemotherapy in bone and soft tissue sarcomas. Before clinical study, we must know by in vitro study whether PEMF affects the cause of malignancy or not. Therefore we examined the effect of PEMF on invasion ability which correlates well with metastasis. The invasion through artificial basement membrane was quantified by invasion assay in a Boyden chamber. In all experiments, there was no difference between PEMF treated group and control. Thus we conclude that invasion ability is not affected by PEMF exposure in vitro”.
In a study published in the April 2011 Journal of Bioelectromagnetics, Zhang D, Pan X et Al. of the Department of Orthopaedic Surgery, Aichi Medical University School of Medicine, Nagakute, Aich, Japan and the Department of Orthopaedic Surgery, the First Hospital, Jilin University, Changchun, China, studied the effects of PEMF on the expression of cell adhesion molecules and matrix metalloproteinase in osteosarcoma cell lines. “Pulsed electromagnetic fields (PEMF) could enhance the cytocidal effects of chemotherapeutic drugs on malignant tumor cell lines, but metastasis effects of PEMF on tumor cells have not been investigated”. The effects PEMF exposure on the expression levels of some metastasis-related molecules: integrin α subunits (α1, α2, α3, α4, α5, α6, αv), integrin β subunits (β1, β2, β3, β4), CD44, and matrix metalloproteinase -2/9 (MMP-2/9) was studied in four human osteosarcoma cell lines. They conclude: “PEMF exposure has no effect on the expression of some molecules associated with tumor cell invasion and metastasis suggesting that PEMF exposure may be safely applied to chemotherapy for osteosarcoma”.
-
Cancer and the Immune System
An increase in cell membrane permeability that promotes more efficient cellular function may also stimulate the immune response to cancer and help the immune system prevent its evolution.
In a study entitled “Immuno-corrective effect of alternating magnetic field in the post operative period in malignant bladder cancer” published in the 2001 Vopr Onkol. Journal, Zlatnik EIu et Al. of the Research Institute of Oncology, Ministry of Health of the RF, Rostov-on-Don, Russia state: ”The study deals with immune status of patients operated for bladder cancer and exposed postoperatively to alternating magnetic field (MF) (hypothalamus and operative field). MF application was followed by higher T - and B-lymphocyte and CD4+, CD16+ cell levels as well as enhanced T - cell activity; no postoperative complications were registered and tumor relapse rates were relatively l ow. The procedure may substitute drug therapy for immuno - correction and prevent recurrence of bladder cancer”.
-
Cancer and Drug Absorption
Many In Vitro studies on drug resistant human cell lines and In Vivo studies, mostly on rodents, prove that PEMF as adjunct therapy enhances the effects of chemotherapy.
In December 2002, the journal Bio electromagnetics published a study on the “Influence of 1 and 25 Hz, 1.5 mT magnetic fields on antitumor drug potency in a human adenocarcinoma cell line” by Ruiz-Gómez MJ et Al. of the Faculty of Medicine, University of Málaga, Spain. The resistance of tumor cells to antineoplastic agents is a major obstacle during cancer chemotherapy. “Many authors have observed that some exposure protocols to PEMF can alter the efficacy of anticancer drugs; nevertheless, the observations are not clear”. They evaluated whether a group of PEMF pulses produces alterations of drug potency on a multidrug resistant human colon adenocarcinoma cell line, HCA-2/1(cch).
The experiments performed included:
a) Drug and PEMF exposure simultaneously for 1 h,
b) Drug exposure for 1 h followed by 2 h/day PEMF exposure for the next 2 days.
Drugs used were Vincristine, Mitomycin C and Cisplatin. The cells were either treated with PEMF or sham exposed. Cell viability was measured by the neutral red stain cytotoxicity test.
The results obtained were:
a) The 1 Hz PEMF increased Vincristine cytotoxicity exhibited a 6.1% survival for which sham exposed groups showed a 19.8% of survival. Treatment with Mitomycin C yielded a 5.3% survival rate as opposed to 19.2% in sham exposed group. Cisplatin showed a significant reduction in the % of survival from 44.2% to 39.1% in the sham exposed group.
b) Minor significant alterations were observed after non - simultaneous exposure of cells to PEMF and drug.
They conclude:
“The data indicate that PEMF can induce modulation of cytostatic agents in HCA -2/1(cch), with an increased effect when PEMF was applied at the same time as the drug. The type of drug, dose, frequency and duration of PEMF exposure could influence this modulation”.
A study by Omote Y et Al. of the Laboratory of Pathology, Hokkaido University School of Medicine, Sapporo, Japan, published in the September 1990 Japanese Journal of Cancer Research investigates the “Treatment of experimental tumors with a combination of a pulsing magnetic field and an antitumor drug”. The effects of a combination treatment with PEMF and an antitumor drug, Mitomycin C, on two experimental tumors (fibro sarcoma KMT-17 and hepatocellular carcinoma KDH-8) in WKA rats were studied. On day 7 after tumor implantation into the right thighs of rats, the region of the tumor was either exposed to PEMF, or not, for 1 h immediately after IV injection of Mitomycin C, or not. Survival rates at day 90 of KMT-17 implanted rats were 0% (0/10) in the non-treated group, 34% (4/12) in the Mitomycin C-treated group, 47% (6/13) in the PEMF-treated group and 77% (10/13) in the Mitomycin C /PEMF combination group. The increase of life span (ILS) of KDH-8-implanted rats in the combination therapy group was significantly prolonged (%ILS 17.6%) compared with that in the Mitomycin C -treated (%ILS 3.4%) and PEMF-treated (%ILS 7.6%) groups. They conclude: “The present results indicate that PEMF exhibited a potentiation of the antitumor effect of Mitomycin C”.
Cancer Tumor and Hypoxia
The American Association for Cancer Research published a study in 2004: “Daily PEMF therapy inhibits tumor angiogenesis via the hypoxia driven pathway: therapeutic implications” by Ivan L. Cameron et Al. of the UT Health Science Center, San Antonio, TX, the Pennington Biomedical Research Center, LSU, Baton Rouge, LA, and EMF Therapeutics, Inc., Signal Mountain, TN. Ten minute daily PEMF was found to retard angiogenesis and growth of a human breast cancer xenograft causing the tumor to develop proportionately larger areas of necrosis and hypoxia and smaller areas of proliferatively active cancer ce lls. Daily PEMF therapy continued to inhibit tumor angiogenesis and tumor regrowth for two weeks following a standard course of ionizing radiation (IR) therapy. However this PEMF therapy renders larger areas of the tumor hypoxic and is expected to lessen susceptibility to oxidative damage caused by further IR treatments or by oxidative dependent chemotherapy. This leads to the conclusion that PEMF is an effective adjunct therapy following IR therapy. However, continued daily PEMF therapy should be stopped sometime (perhaps 2-4 days) prior to a second round of IR therapy for resumption of angiogenesis, decrease of hypoxic areas, an increase in proliferative activity and well oxygenated areas within the tumor for the second IR to be effective.
A later study by Cameron I.L. et Al. published in the July 2005 Cancer Cell International confirms the effectiveness of PEMF in combination with gamma radiation to control tumor development in mice injected with human breast cancer xenograft. A variation of PEMF therapy was used in the study “Therapeutic Electromagnetic Field (TEMF) and gamma irradiation on human breast cancer xenograft growth, angiogenesis and metastasis”. The mice were divided in four groups: a control group, a group treated with gamma radiation only, a group treated with TEMF only and a group treated with a combination of both gamma radiation and TEMF. Cameron et Al. conclude: “Mice that received either IR or TEMF had significantly fewer lung metastatic sites and slower tumor growth than did untreated mice. No harmful side effects were attributed to TEMF. TEMF therapy provided a safe means for retarding tumor vascularization, growth and metastasis”. Although no In Vivo human studies have been conducted thus far on the use of PEMF as adjunct therapy to the traditional cancer and tumor treatments, the many studies conducted In Vitro on tissue cultures and In Vivo studies on rodents show promise of better results with no known adverse side effects to date.
PEMF Therapy Increases Cellular Metabolism
In a study on Chronic Fatigue Syndrome and Electro - medicine, Thomas Valone, Ph.D, showed that damaged or diseased cells present an abnormally low TMP, about 80% lower than healthy cells. This signifies a greatly reduced metabolism and, in particular, impairment of the electrogenic Na+/ K+ pump activity associated with reduced ATP (Adenosine Tri-Phosphate) production.
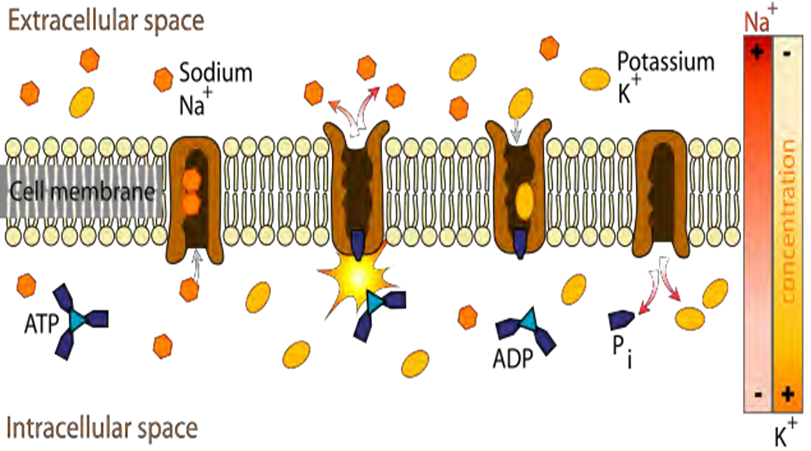
For proper metabolism, the Na+/ K+ pump within the membrane forces a ratio of 3Na+ ions out of the cell for every 2K+ ions pumped in. The Na+/ K+ pump uses energy derived from ATP to exchange Na+ for K+ ions across the membrane.
An impaired Na+/ K+ pump results in edema (cellular water accumulation) and fermentation, a condition favorable to cancerous activity. French researcher Louis C. Kervran demonstrated that Sodium plus Oxygen plus Energy (ex: magnetic energy) nuclearly transmutes into Potassium as
11 Na23 + 8 O16 + energy = 19 K39
As a result, utilization of oxygen in the cells increases and the body increases production of its own energy supplier (ATP). The organism becomes more stable and efficient; toxins and waste products are more rapidly broken down. The body's natural regulatory mechanisms are reinforced and healing processes accelerated. Free radical proliferation cause cellular malfunction or mutation (i.e. cancer) and protein degradation.
Free radicals cause damage to all cells of the body and to the immune system. Free radicals deplete cellular energy by interfering with mitochondrial function contributing to a shortened lifespan. Cellular energy generation in the mitochondria is both a key source and a key target of oxidative stress in cells. To complete the radical, free radicals rip electrons from molecules and cause chain reactions creating other free radicals. Antioxidants such as vitamin A, vitamin E, selenium and coenzyme Q10 supply free electrons and are usually prescribed to provide limited relief counteracting free radical ravages. However, PEMF therapy can also satisfy and terminate free radicals by abundantly supplying the key ingredient usually found only in encapsulated anti-oxidant supplements…the electron (Thomas Valone, Ph.D. on Bioelectromagnetics, 2003 ).
In 1976, Nobel Prize winner Dr. Albert Szent -Gyorgi established that structured proteins behave like diodes or rectifiers. A diode passes electricity in only one direction. Dr. Szent-Gyorgi proposed that cell membranes can rectify an induced voltage causing changes in the ion concentration of the inner and outer surfaces of the cell membrane in such a way as to increase the TMP and effectively stimulate the activity of the Na+/ K+ pump. Cell health is directly affected by the health of the Na+/ K+ pump, which is directly proportional to the TMP.
Based on these biophysical principles, a high voltage EMF of sufficient strength will theoretically stimulate the TMP, normal cell metabolism, the Na+/ K+ pump, ATP production and healing. Dr. Albert Szent-Gyorgi summarizes: “TMP is proportional to the activity of this pump and thus to the rate of healing. Increases in the TMP have also been found to increase the uptake of amino acids.” This is important, as an increased supply of nutrients promotes cell repair.
PEMF Therapy Increases Energy Storage and Cellular Activity
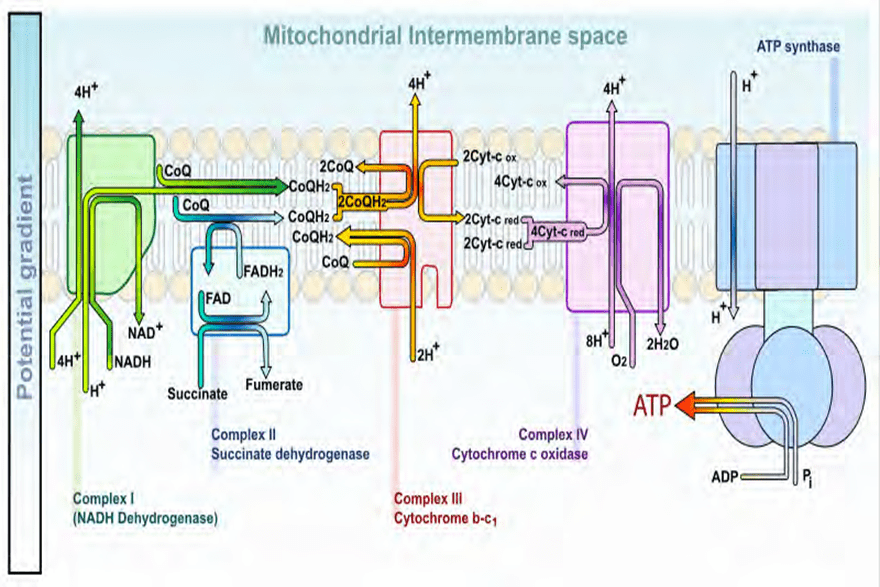
At the sub-atomic level, as the PEMFs expand and collapse through a tissue, the protein molecules, such as the cytochromes in mitochondria, gain electrons and, in doing so, store energy. Although the instantaneous peak magnetic energy amplitudes are very high, the average magnetic amplitudes generated by PEMF therapy remain low. The average total energy transmitted to the tissues is not powerful enough to create heat within the cells, nor for the cells’ atoms to vibrate sufficiently to cause a thermal increase or an electron to jump to a higher orbit and emit heat as it returns to its orbit of origin. There is only sufficient average energy for the electron-spin to be increased. Thus, energy gets stored in the cells’ mitochondria by converting ADP (Adenosine Di-Phosphate) to ATP (Adenosine Tri-Phosphate) molecules more rapidly. The ATP molecules store and transport th e energy that is then used in the many chemical processes within the cell that participate in all the metabolic functions. This phenomenon is referred to as the electron transport chain and is described in the diagrams below:
Understanding the effects of PEMF therapy at the atomic level requires a basic understanding of Quantum Mechanics. The Schrödinger equation for a molecule calculates the probable amplitude for its electrons over an infinite number of possible trajectories and yields the vibrational states of the molecule. This defines how the quantum state or wave function of a molecule or physical system changes in time. A diatomic molecule, which has only one vibrational degree of freedom, (the stretching of the bond between the electron and the positon) provides a simple description (Atkins et Al. 2002).
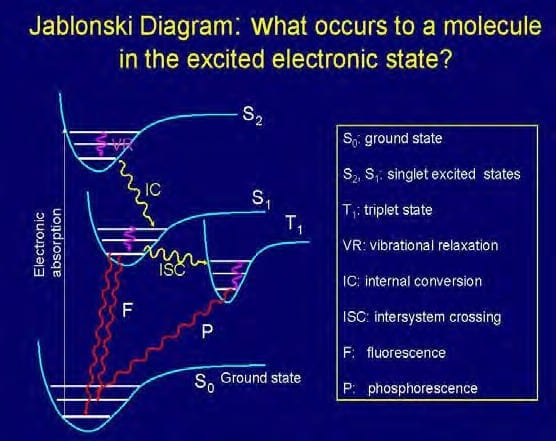
Quantum mechanical considerations show that during the electronic excitation of a particular molecule at the same orbital state, the energy of an excited triplet state (T1) , where 2 electrons are unpaired, is lower than that of its corresponding singlet state (S1). In biomolecules, the non-radiative crossing from the state S2 to S1 is generally the dominant mechanism. This crossing between two electronic states of the same spin multiplicity is called internal conversion (“IC”). The IC process is then followed by a rapid vibrational relaxation (decrease) where the excess vibrational energy is dissipated into heat, the molecule now ending up at the lowest, zero-point vibrational level of the S1 electronic state. (Atkins et Al. 2002). From here, it can return to the ground electronic state S0 by emitting a photon (radiatively).
The time-varying magnetic fields associated with PEMF therapy apparently affect electronic states via the intercrossing system (“ISC”), which is an e xcitation from state Si to Ti, where Ti is the corresponding triplet state. In shifting positions around an atomic nucleus, an electron generates energy and emits a magnetic resonance of specific frequency. Thus, the magnetic resonance field frequency of the various body tissues and organs is a product of the individual atomic, molecular and cellular frequencies specific to the molecules that constitute the particular tissue or organ.
PEMF therapy provides sufficient energy to affect the magnetic resonance of the atom as the electron is energized. The magnetic resonance of the electrons at the atomic level also exhibits a change, a phase shift that disturbs and breaks the once orderly pathways of communication that is usually transmitted from atom to molecule, molecule to cell, cell to tissue, and tissue to organ. PEMF seems to confuse the specific inherent magnetic resonance and temporarily modify it in each atom, molecule, cell, and thus, tissue and organ.
In doing so, the phase shift influences the physical and chemical characteristics of the physiological markers.
PEMF therapy has proven beneficial in many ways for the various energetic body functions.
All of the many types of living cells that make up the tissues and organs of the body are tiny electrochemical units. They are powered by a "battery” that is continually recharged by the cells' metabolic chemistry in a closed loop of biological energy.
PEMF Therapy Increases Cell Membrane Flexibility and Elasticity
A study entitled “Modulation of collag en production in cultured fibroblasts by a low -frequency pulsed magnetic field” by Murray et Al. (Biochim Biophys Acta) shows that total protein synthesis was increased in confluent cells treated with a PEMF for the last 24 h of culture as well as in cells treated for a total of 6 days. However, in 6 day-treated cultures, collagen accumulation was specifically enhanced as compared to total protein, whereas after short-term exposure, collagen production was increased only to the same extent as total protein. These results indicate that a PEMF can specifically increase collagen production, the major differentiated function of fibroblasts.
PEMF therapy successfully increases membrane flexibility by stimulating the synthesis of collagen in the fibroblasts, a crucial protein for membrane elasticity. In doing so, PEMF therapy increases tissue and muscle flexibility and, thus, increases range of motion.
PEMF Therapy Stimulates Cellular Communication and Replication
DNA synthesis is linked to pulsed, low intensity magnetic fields (Liboff et Al. 1984; Rosch et Al. 2004). Proteins are conductors of electricity. When exposed to strong fields, proteins are subject to electrophoresis. Ribonucleic Acid (“RNA”) messengers are synthesized from a Deoxyribonucleic Acid (“DNA”) template during transcription. They transfer genetic information from the cell nucleus to ribosomes in the cytoplasm and serve as a template for protein synthesis. Because of the mechanical influences between the RNA, the DNA and encoded proteins, the flow of information to and from genes may be linked to changing magnetic fields (Einstein, 1977; Goodman et Al.1983).
Since magnetic fields interact with changing electrical charges and recent studies (Dandliker et Al.1997) show that DNA conducts electrons along the stacked bases within the DNA double helix, electro-magnetic fields may initiate transcription of the precursor mRNA by accelerating electrons moving within the DNA helix (McLean et Al. 2003).
PEMF Therapy Increases Cellular Genesis (Cellular Growth and Repair)
The many intra and inter cellular processes and activity stimulated by PEMF therapy lead to faster cellular and tissue regeneration. This fact is shown by the results of many studies on a variety of tissues, including bones, spine, cartilage, intestines, blood vessels, nerves, brain, and muscles.
In December 2004, the Swiss Medical Tribune stated that PEMF therapy provided: “improvement of blood circulation, relief from pain, improvement of bone healing and the stimulation of nerve cells. Not only is the PEMF therapy effective in disease condition: it is an excellent means of preventing stress, assisting regeneration and recovery after sports exertion… Through metabolic activation and blood circulation more nutrients and oxygen are available to muscle cells, less damage is experienced, and efficiency is improved.”
PEMF and the spine
In a long-term study entitled: “Spine fusion for discogenic low back pain: outcome in patients treated with or without pulsed electromagnetic field stimulation”, Marks RA. (Richardson Orthopaedic Surgery, TX, USA) randomly selected 61 patients who underwent lumbar fusion surgeries for discogenic low back pain between 1987 and 1994 and had failed to respond to preoperative conservative treatments. Average follow - up time was 15.6 months postoperatively. Fusion succeeded in 97.6% of the 42 patients who received PEMF stimulation for only 52.6% of the 19 patients who did not receive electrical stimulation of any kind.
A similar study by Richard A. Silver, M.D. (Tucson Orthopaedic & Fracture Surgery Associates, Ltd., Tucson, AZ, USA) with 85 patients who had undergone surgery of posterior lumbar interbody fusion (PLIF) and had risk factors associated with a poor prognosis for healing, including smoking, prior back surgery, multiple spinal levels fused, diabetes millitus, and obesity, roentgenographic examination and clinical evidence indicated that all but two patients achieved successful fusion. Of the 83 patients with successful spinal fusion, 29 (34.9%) were assessed as "excellent," 45 (54.2%) as "good," 3 (3.6%) as "fair", and 6 (7.2%) as "poor". Adjunctive treatment with PEMF appeared effective in promoting spinal fusion following PLIF procedures across all patient subgroups.
PEMF, cartilage and bones
In a study entitled: “Modification of biological behavior of cells by Pulsing Electro -magnetic fields”, Ben Philipson, Curatronic Ltd. (University of Hawaii School of Medicine, HI, USA) treated 20 subjects, ages 57 to 75 years, with decreased bone mineral density a s defined by a bone densitometer, with PEMF therapy for a period of 12 weeks. After 6 weeks, the bone density rose by an average of 5.6%. Pulsed electromagnetic fields appliedto the whole body have clear clinical benefits in the treatment of bone diseases, often micro-fractures in vertebrae and related pain. In addition, joint pain from worn out cartilage layers can be treated successfully with electromagnetic stimulation. PEMF application promotes bone union by electric current induction with an increase in cell membrane permeability and stimulation of intracellular cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) activity. Osteoblast differentiation accelerates by the activation of p38 phosphorylation.
In healthy joints, movement generates electric currents in the joint. The electrical field protects and regenerates the cartilage, surrounding bone and connective tissues (tendons, ligaments...). In the joint space, chondrocytes produce a cartilaginous extracellular matrix that supports and increases the space between individual cells. This matrix consists mostly of collagen type II and aggrecans.
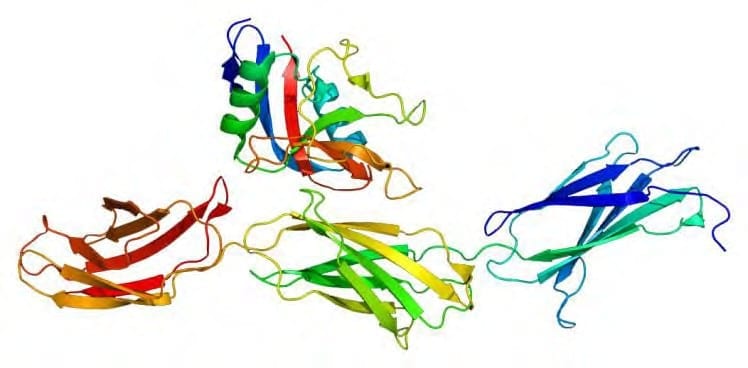
An aggrecan is a chondroitin sulfate proteoglycan 1 that consists of two globular structural domains, a chondroitin sulfate G1 and a keratan sulfate G2, around a protein core, a large globular domain G3 with 100 to 150 glycosaminoglycan GAG chains. Keratan sulfates are large, highly hydrated molecules which act as a cushion to absorb mechanical shock. GAGs are highly negatively charged molecules that impart high viscosity to the solution. With their high viscosity, GAGs have low compressibility and lubricate the joints. Injuries and osteoarthritis alter the electrical field in the joint and thus disrupt the normal mechanism of preservation and regeneration of the cartilage.
The ability of the cartilaginous tissue to withstand compressive stress is reduced. Once the destructive process has begun and the delicate integrity of healthy resting chondrocytes in maturational arrest is disturbed, phenotypic dedifferentiation of the chondrocytes may become hypertrophic. In the hypertrophic state, the chondrocytes start to lose their cartilage -specific phenotype and produce harmful cartilage matrix degrading enzymes like MMPs and aggrecanases in pathological quantities. This process hinders the production of proteoglycans, GAGs and chondroitin sulfate increasing the ratio of keratan to chondroitin sulfate reducing hydration and reducing the resistance of the amorphous joint fluid. The collagen fibrils are then subjected to intolerable mechanical pressure. The cavities created by enzymatic destruction are filled with hypertrophic chondrocytes having bone-specific collagen type I and X. The chondrocytes become apoptotic causing a calcification of the matrix that generates matured bone. As such, chondrocyte hypertrophy debilitates cartilage viability with bone- specific collagens, which initiates calcification of the matrix and finally programmed chondrocyte cell death.
In a study entitled: “Effects of pulsed electromagnetic fields on patients” recovery after arthroscopic surgery: a prospective, randomized and double-blind study” conducted at the "Sacro Cuore Don Calabria" Hospital, Negrar (Vr), Italy, and published in the Knee Surgery , Sports Traumatology, Arthroscopy journal in July 2007, Zorzi C et Al. state: “Severe joint inflammation following trauma, arthroscopic surgery or infection can damage articular cartilage, thus every effort should be made to protect cartilage from the catabolic effects of pro - inflammatory cytokines and stimulate cartilage anabolic activities. Previous pre-clinical studies have shown that PEMFs can protect articular cartilage from such catabolic effects and prevent its degeneration resulting in chondroprotection. This prospective study was to evaluate the effects of PEMFs in patients undergoing arthroscopic treatment of knee cartilage. They were randomized into two groups: a control group (magnetic field at 0.05 mT) and an active group (magnetic field of 1.5 mT). All patients used PEMFs for 90 days, 6 h per day. The patients were evaluated by the Knee injury and Osteoarthritis Outcome Score (KOOS) test before arthroscopy, after 45 and after 90 days. The use of non - steroidal anti-inflammatory drugs (NSAIDs) to control pain was also recorded. Patients were interviewed for the long-term outcome, 3 years after arthroscopic surgery. Thirty-one patients completed the treatment. KOOS values at 45 and 90 days were higher in the active group and the difference was significant at 90 days (P < 0.05). The percentage of patients who used NSAIDs was 26% in the active group and 75% in the control group (P = 0.015). At the 3 years follow-up, the number of patients who completely recovered was higher in the active group compared to the control group (P < 0.05). Treatment with PEMF aided patient recovery after arthroscopic surgery, reduced the use of NSAID and had a positive long-term effect.
Many studies demonstrate that PEMF stimulation protects cartilage from degeneration. PEMF increases blood and fluid flow, and thus, the partial oxygen pressure in the cells and in the joint space as well as ion transport in the chondrocytes. This phenomenon helps maintain the chondroitin / keratan sulfate proteoglycan balance and levels of glycosaminoglycan in the matrix. Repair and growth of cartilage is then stimulated preventing further degradation and the grinding of the bones.
PEMF and tendons
The Department of Rheumatology at Addenbrookes Hospital carried out investigations into the use of PEMF therapy for the treatment of persistent rotator cuff tendonitis. PEMF treatment was applied to patients who had symptoms refractory to steroid injection and other conventional treatments. At the end of the trial, 65% were symptom free and 18% greatly improved.
In a study entitled “Pulsed Magnetic Field Therapy Increases Tensile Strength in a Rat Achilles’ Tendon Repair Model” published in 2006 ( Dept. of Plastic and Reconstructive Surgery, Albert Einstein College of Medicine and Montefiore Medical Center, Bronx, NY; Dept. of Biomedical Engineering, Columbia University, New York, NY; and Dept. of Orthopaedics, Mount Sinai School of Medicine, New York, NY. ), Berish Strauch, MD. et Al. conclude: “The use of electromagnetic fields in tissue healing is still a relatively recent application and much research remains to be performed. Areas that need greater explanation include the interplay between wound healing, contributing growth factors and angiogenesis. PEMFs hold promise as a safe, easily administered and noninvasive modality to accelerate and improve the body’s healing mechanisms”.
PEMF and intestines
An experimental study designed by Nayci et Al. (Dept. of Pediatric Surgery, Mersin University Medical Faculty, Turkey) investigated the effect of PEMF therapy on intestinal healing, and compared small and large intestinal anastomoses, connections between the loops of the intestines. The study demonstrated that PEMF stimulation provides a significant gain in anastomotic healing in both small and large intestines, and increases in both biochemical and mechanical parameters.
PEMF and the brain
A four-week double-blind, placebo-controlled study conducted by Uni der Bundeswehr (Munich, Germany) assessed the “Efficacy of PEMF Therapy for Insomnia”. One hundred one patients were randomly assigned to either active treatment (n = 50) or placebo (n = 51) , and to one of three diagnostic groups: sleep latency; interrupted sleep; or nightmares. The results showed 70% (n = 34) of the patients given active PEMF treatment experienced substantial or even complete relief of their complaints; 24% (n = 12) reported clear improvement; 6% (n = 3) noted a slight improvement. Only one placebo patient (2%) had very clear relief; 49% (n = 23) reported slight or clear improvement; and 49% (n = 23) saw no change in their symptoms. No adverse effects of treatment were reported.
Stunning results were obtained in a study entitled : “Protection against focal cerebral ischemia following exposure to a pulsed electro -magnetic field, Grant G et Al. (1994 Dept. of Neurosurgery, Stanford University, CA, USA) stated: “There is evidence that electromagnetic stimulation may accelerate the healing of tissue damage following ische mia. Exposure to PEMF attenuated cortical ischemia edema on MRI at the most anterior coronal level by 65%. On histological examination, PEMF exposure reduced ischemic neuronal damage in this same cortical area by 69% and by 43% in the striatum. Preliminary data suggest that PEMF treatment of short duration may have implications for the treatment of acute stroke”.
PEMF and nerves
In 2003, Thomas J. Goodwin, Ph.D. of the NASA Lyndon B. Johnson Space Center, Houston, Texas, published a study on the “Physiological Effects of Time - Varying Electromagnetic Fields (TVEMF) on Human Neuronal Cells” based on model systems for growing two- (2D) and three-dimensional (3D) human neural progenitor cells. “Neuronal tissue comprises elongated nerve cells composed of elongated axons, dendrites and nuclear areas. Axons and dendrites are chiefly responsible for transmission of neural signals over distance. Longitudinal cell orientation is critical f or proper tissue formation and function, including growth”. The same electromagnetic field strength and all other conditions were identical for the 2D and 3D in vitro experimental conditions with the 3D cultures exposed to microgravity using a rotating wall vessel (RWV). The study showed that extremely low amplitude rapidly TVEMF of 10 to 200 mGauss (below the Earth’s field strength of 500 mGauss) exert a very potent effect on the proliferation, morphology and gene expression of the cells in both the 2D and 3D cultures as well as organization into 3D clusters in the RWV. This electromagnetic potentiation can be used for a number of purposes including the development of tissues for transplantation, repair traumatized tissues, moderate some neurodegenerative diseases and perhaps control the degeneration of tissue as might be effected in a bioelectric stasis field.
PEMF and multiple sclerosis
At the Biologic Effects of Light 1998 Symposium, Richards et Al. (Dept. Radiology, University of Washington, WA, USA) explain the effects of pulsing magnetic field on brain electrical activity in multiple sclerosis (MS): “MS is a disease of the central nervous system. Clinical symptoms include fatigue, impaired bladder control, muscle weakness, sensory deficits, impaired cognition, and more. Histologic, immunologic, and radiologic studies show there are demyelinated brain lesions (visible on MRI) that contain immune cells such as macrophages and T-cells (visible on microscopic analysis of brain sections). We recently published a review entitled "Bio-electromagnetic applications for MS" which examined several scientific studies demonstrating the effects of PEMFs on nerve regeneration, brain electrical activity (electro - encephalography EEG), neurochemistry and immune system components”.
Richards referred to a study that evaluated EEG in response to photic stimulation with flashing lights before and after PEMF exposure. The data showed a significant increase in alpha EEG magnitude that was greater in the active compared to the placebo group with increased activity. Richards et Al. confirm the above conclusion in a double-blind study to measure the clinical and sub-clinical effects of an electromagnetic device on disease activity. The MS patients were exposed to a device that was ei ther active (PEMF) or inactive (placebo) for two months. Each MS patient received a set of tests to evaluate MS disease status before and after wearing the device. The tests included a clinical rating (Kurtzke, EDSS), patient reported performance scales (PS), and quantitative EEG (QEEG) during a language task. Although no significant change was noted between pre and post-treatment in the EDSS scale, there was a significant improvement in the PS combined rating for bladder control, cognitive function, fatigue level, mobility, spasticity, and vision. There was also a significant change between pre -treatment and post-treatment in alpha EEG magnitude. Richards et Al. stated: “we have demonstrated a statistically significant effect of the magnetic pulsing device on patient performance scales and on alpha EEG magnitude during a language task”.
In “Treatment with AC PEMFs normalizes the latency of the visual evoked response in a MS patient with optic atrophy”, Sandyk (Dept. of Neuroscience at the Institute for Biomedical Engineering and Rehabilitation Services of Touro College, Dix Hills, NY, USA, 1998) explains: “Visual evoked response (VER) studies have been utilized as supportive information for the diagnosis of MS and may be useful in objectively monitoring the effects of various therapeutic modalities. Delayed latency of the VER, which reflects slowed impulse transmission in the optic pathways, is the most characteristic abnormality associated with the disease. Brief transcranial applications of AC PEMFs in the picotesla flux density are efficacious in the symptomatic treatment of MS and may also reestablish impulse transmission in the optic pathways… The rapid improvement in vision coupled with the normalization of the VER latency despite the presence of optic atrophy from chronic demyelization of the optic nerve, cannot be explained on the basis of partial or full reformation of myelin. MS synaptic neurotransmitter deficiency is associated with the visual impairment and delayed VER latency following optic neuritis and the recovery of the VER latency by treatment with PEMFs is related to enhancement of synaptic neurotransmitter functions in the retina and central optic pathways. Recovery of the VER latency in MS patients may have important implications with respect to the treatment of visual impairment and prevention of visual loss. Specifically, repeated applications of PEMFs may maintain impulse transmission in the optic nerve and thus potentially sustain its viability”.
Sandyk R. summarizes recent clinical work on the therapeutic effects of AC PEMF in MS : “MS is the third most common cause of severe disability in patients between the ages of 15 and 50 years. No specific treatment modality can cure the disease or alter its long-term course and eventual outcome. Moreover, there are no agents or treatments that will restore premorbid neuronal function. A host of biological phenomena associated with the disease cannot be explained on the basis of demyelization alone and require refocusing attention on alternative explanations, one of which implicates the pineal gland. The pineal gland acts as a magneto-receptor organ. This biological property of the gland provided the impetus for the development of a novel and highly effective therapeutic modality, which involves transcranial applications of alternating current AC PEMFs flux density” (1997).
Summary
Beyond its complex mechanisms, PEMF therapy offers many health benefits. PEMFs help the natural body healing processes by delivering a non -invasive form of repetitive electromagnetic stimulation that requires no direct contact with the skin surface. PEMF treatment stimulates many aspects of cellular metabolism and activity through a chain of processes in the human body that lead to health improvement without side effects.
The many studies cited here demonstrate that magnetic fields affect many biologic processes and are effective in a wide range of medical conditions.
Through these processes, vital organs such as the liver, kidneys and colon function better and eliminate toxins more efficiently and thus help the body detoxify. PEMF treatment promotes cellular genesis with cartilage and bone formation, fracture and osteotomy repair, healthier joints, greater bone density, tendon and soft tissue repair with better recovery from wounds and trauma, graft and surgery. PEMF improves urinary incontinence, osteoarthritis, impaired neural function and central nervous system diseases such as MS and spinal cord damage. PEMF therapy improves sports performance and simply helps to maintain good health, promote healing and a return to better function and higher activity levels. PEMF therapy is an invaluable aid to the clinic. PEMF therapy leaves you feeling relaxed, energized, renewed and with a sense of well -being.
Thank you to Wikipedia English for public access to its formidable scientific data resources.